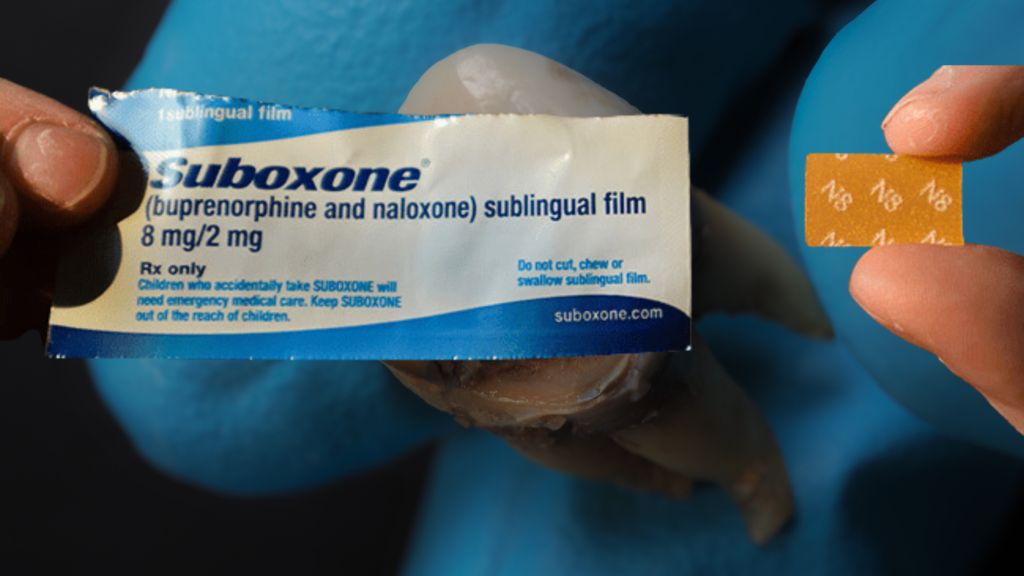
Suboxone Film Lawsuit - How You Qualify:
- Used name brand Suboxone sublingual film (Generic released 6/2018)
- Dental problems resulting in at least 3 or more teeth being broken, lost or extracted after using Suboxone sublingual film
Suboxone Lawsuit - Main Allegations:
The Suboxone lawsuit alleges that the manufacturer of Suboxone failed to provide adequate warnings about the risk of dental problems associated with Suboxone sublingual film. The Suboxone attorneys argue that the company prioritized profits over patient safety.
important!
Suboxone Lawsuit Claims Take Time To Investigate And File - Contact Us Now So We Can Preserve Your Suboxone Claim!
SUBOXONE LAWSUIT CONSULTATION HOTLINE
Suboxone Lawsuit: The Hidden Danger of Dental Issues
Suboxone Takes Over The Opioid Addiction Treatment Market
Suboxone was originally approved for sale in 2002 as a tablet to be administered under the tongue and then in 2010 Suboxone was approved as a film to be placed inside the cheek to treat pain. Suboxone has been praised for its effectiveness in treating opioid addiction treatment, even though cheaper alternatives exist, such as Methadone. Even though Suboxone is more expensive, Suboxone was marketed as having fewer side effects than Methadone and Suboxone can be obtained with a prescription instead of having to go to a clinic like with Methadone. As a result, over 16 million Suboxone prescriptions are filled every year resulting in annual Suboxone sales exceeding $1 billion annually! As expected, Suboxone use is significantly high in states that were hit hardest by the opioid epidemic.
- States with the highest Suboxone prescriptions per 100 persons are West Virginia (27), Vermont (25), Kentucky (23), and Main (18).
- States with the lowest Suboxone prescriptions per 100 persons are Iowa (1.3), Teas (1.4), California (1.6), and Hawaii (1.8).
Suboxone’s Alarming Side Effect: Dental Problems
Suboxone has recently faced a storm of controversy as the drug’s hidden side effects became evident. An increasing number of severe dental side effects reports have cast a shadow over its widespread use, culminating in Suboxone lawsuits against the drug’s manufacturer – Indivior. Many Suboxone film users are reporting that many and in some cases all of their teeth have broken and required extraction. Even the FDA has taken note of the dental issues associated with using Suboxone film, but it appears that the FDA is only aware of the tip of the iceberg when it comes to the number of individuals who have experienced dental side effects from using Suboxone Film.
FDA Warns Consumers About Dental Problems with Suboxone Dissolved in the Mouth
On January 12, 2022, the FDA issued a drug safety communication regarding Suboxone film. The FDA issued the following warning regarding Suboxone:
- “The U.S. Food and Drug Administration (FDA) is warning that dental problems have been reported with medicines containing buprenorphine [Suboxone] that are dissolved in the mouth. The dental problems, including tooth decay, cavities, oral infections, and loss of teeth, can be serious and have been reported even in patients with no history of dental issues.”
The FDA went on to note that the dental side effects from Suboxone only occur in the versions of Suboxone that require the user to hold the drug in their mouth. The FDA said that Suboxone patches and injections do not pose a risk of causing dental problems as Suboxone sublingual film does.
The FDA warned that to reduce the dental side effects associated with Suboxone that “patients using [Suboxone] dissolved in the mouth should take extra steps to help lessen the risk of serious dental problems. After the [Suboxone] is completely dissolved, take a large sip of water, swish it gently around your teeth and gums, and swallow. You should wait at least 1 hour before brushing your teeth to avoid damage to your teeth and give your mouth a chance to return to its natural state.”
The FDA went on to advise those taking Suboxone to “inform your health care professional if you have a history of tooth problems, including cavities. Schedule a dentist visit soon after starting this medicine and inform your dentist that you are taking [Suboxone], and schedule regular dental checkups while taking [Suboxone].”
Why Did The FDA Warn About Suboxone Causing Dental Problems?
The FDA stated that since Suboxone was approved in 2002, the FDA identified 305 cases of dental problems associated with Suboxone medicines dissolved in the mouth. Of the 305 reported cases of dental problems associated with Suboxone dissolved in the mouth, 131 of those cases were classified as serious dental issues (impacted two or more teeth). Furthermore, 26 cases had no prior history of dental problems prior to using Suboxone. Dental problems occurred as soon as 2 weeks after beginning treatment with Suboxone, with the median time to diagnosis of dental problems being 2 years after starting treatment with Suboxone.
The FDA noted that the reports of dental problems caused by Suboxone only came from published medical literature or adverse events that were directly reported to the FDA. The FDA admitted that there were likely cases of Suboxone causing tooth decay that the FDA is currently unaware of. Our Suboxone attorneys believe that 10’s of thousands of individuals have suffered dental problems caused by Suboxone, but most doctors and patients are completely unaware of the association between tooth decay and Suboxone.
The FDA also noted that many of the adverse events of dental problems related to Suboxone use were reported by health care professionals who provided documentation of “extensive dental adverse events.” The FDA found that the most common treatment for dental problems associated with Suboxone use was tooth extraction / tooth removal. As of 2022, the FDA was aware of 71 patients who had undergone tooth extraction or tooth removal after starting Suboxone. Other Suboxone adverse events reported to the FDA included root canals, dental surgery, and other procedures such as crowns and implants.
FDA Suboxone Warning to Health Care Professionals Regarding
The FDA provided the following additional warning regarding Suboxone to Health Care Professionals:
- “FDA is warning that cases of dental adverse events, some severe, have been reported following the use of transmucosal [Suboxone] medicines. Reported events include cavities/tooth decay, including rampant caries; dental abscesses/infection; tooth erosion; fillings falling out; and, in some cases, total tooth loss. Multiple cases were reported in patients with no prior history of dental problems. The most common treatment for the dental adverse events was tooth extraction/removal.”
Why Suboxone Sublingual Film Increases The Risk Of Tooth Decay And Other Dental Problems
There are several reasons that Suboxone administered sublingually causes adverse effects on the teeth. One of the main issues is that Suboxone is acidic, with a pH of 3.4 when dissolved in water. Next, Suboxone has poor oral bioavailability (it doesn’t absorb in the mouth easily), so the manufacturer advised patients to keep the Suboxone and accumulating saliva in their oral cavity to maximize absorption through the mucosal surfaces. Initially, the manufacturer of Suboxone also advised patients to not rinse their mouth after taking Suboxone. Additionally, many patients take Suboxone three or more times a day. This can result in the Suboxone altering the tooth surface microbial profile and the pH, which then promotes dental caries.
More specifically, sublingual formulations of Suboxone contain citric acid, which can increase the acidity in the mouth and promote demineralization of the teeth. Additionally, Suboxone can reduce the salivary flow or amount of saliva in a person’s mouth, which further promotes tooth decay.
The manufacturer of sublingual Suboxone should have known that Suboxone is extremely acidic. Furthermore, a responsible pharmaceutical company should have been aware that exposing teeth to acidic conditions for a prolonged period of time would cause dental damage. Despite this, the company did not warn patients or physicians that Suboxone could cause damage to patient’s teeth.
Suboxone Label Updated
After the FDA’s Suboxone causing dental problems warning was issued in January of 2022, it took the manufacturer an astounding 6 months to make any updates to the Suboxone label! Once they finally updated the Suboxone label, any new information was buried. Not only was a black box warning not added to the Suboxone label, but nothing on the first page of the label was updated, including the “warnings and precautions” section on the front page. Instead, the first change to the Suboxone instructions for use (IFU) appears on page 5, instructing physicians prescribing Suboxone to:
- “Advise patients to do the following after the product has completely dissolved in the oral mucosa: take a sip of water, swish gently around the teeth and gums, and swallow. Advise patients to wait for at least one hour after taking Suboxone before brushing teeth.”
Finally, buried all the way at the bottom of page 10 of the instructions for use of Suboxone the following section titled “Dental Adverse Events” was added:
- “Cases of dental caries, some severe (i.e., tooth fracture, tooth loss), have been reported following the use of transmucosal [Suboxone]. Reported events include cavities, tooth decay, dental abscess/infection, rampant caries, tooth erosion, fillings falling out, and in some cases, total tooth loss. Treatment for these events included tooth extraction, root canal, dental surgery, as well as other restorative procedures (i.e., fillings, crowns, implants, dentures). Multiple cases were reported in individuals without any prior history of dental problems.
- “Refer patients to dental care services and encourage them to have regular dental checkups while taking Suboxone. Educate patients to seek dental care and strategies to maintain or improve oral health while being treated with [Suboxone]. Strategies include, but are not limited to, gently rinsing the teeth and gums with water and then swallowing after Suboxone has been completely dissolved in the oral mucosa. Advise patients to wait for at least one hour after taking Suboxone before brushing teeth.”
Suboxone Lawsuits Timeline
There have been several criminal and civil investigations regarding Suboxone and the manufacturers of Suboxone, including several very large settlements.
Justice Department Obtains $1.4 Billion Suboxone Related Settlement
In July of 2019, The United States Department of Justice obtained a $1.4 billion settlement from Rickitt Benckiser Group, the parent company of Indivior who manufactures Suboxone, related to the marketing of Suboxone. The $1.4 billion Suboxone settlement was noted to be the largest opioid-related recovery for the United States. The $1.4 billion Suboxone settlement included a forfeiture of proceeds totaling $647 million, civil Suboxone settlements with state and federal governments totaling $700 million, and an administrative resolution with the Federal Trade Commission for another $50 million.
The federal indictment against the manufacturers of Suboxone alleged the manufacturers, “promoted the film version of Suboxone (Suboxone Film) to physicians, pharmacists, Medicaid administrators, and others across the country as less-divertible and less-abusable and safer around children, families, and communities than other buprenorphine drugs, even though such claims have never been established.” Additionally, the makers of Suboxone set up a “Here to Help” internet and telephone program as a resource for opioid-addicted patients. In reality, the “Here to Help” program was set up to direct patients to doctors who the company knew would prescribe them Suboxone. Finally, the makers of Suboxone announced they were going to discontinue the tablet form of Suboxone based on alleged “concerns regarding pediatric exposure” to the Suboxone tablets. However, the primary reason that Suboxone tablets were discontinued was to delay the FDA from approval a generic tablet form of Suboxone – to prevent competition and price erosion of the drug. The scheme to expand Suboxone’s market share and profits was wildly successful.
Suboxone Manufacturer Pleads Guilty to Felony Charge & Agrees to Settle Criminal & Civil Investigations for $600 Million
In July of 2020, Indivior Solutions – the manufacturer of Suboxone, agreed to a $600 million Suboxone settlement with the Department of Justice. That’s a total Suboxone settlement of $2 billion when accounting for the $1.4 billion Suboxone settlement by Indivior’s parent company in 2019. Additionally, the maker of Suboxone admitted to one-count of felony criminal information charging false statements related to health care matters. In pleading guilty, the maker of Suboxone admitted to making false statements to promote the film version of Suboxone (Suboxone Film) to the Massachusetts Medicaid program (MassHealth) relating to the safety of Suboxone Film around children. The following terms were also part of the Suboxone settlement:
- Disband the Suboxone sales force and never reinstate a Suboxone sales force;
- The CEO of the company that makes Suboxone must personally certify annually that they are compliant with the FDA and not committing health care fraud;
- Prohibit the makers of Suboxone from using data obtained from surveys of health care providers for marketing, sales, and promotional purposes;
- Require the makers of Suboxone to remove health care providers from their promotional programs if the provider is at high risk of inappropriate prescribing; and
- Make the manufacturer of Suboxone subject to contempt sanctions by the court and reinstatement of the dismissed charges if it violates the agreement.
Out of the $600 million Suboxone settlement, approximately $209 million of the Suboxone settlement went to the federal government and another $91 million went to state governments. The remainder of the Suboxone settlement was to resolve criminal penalties.
Suboxone Manufacturer Indivior’s Former CEO Sentenced to Jail
In October of 2020, Shaun Thaxter, the former chief executive officer (CEO) of Indivior, the company that makes Suboxone, was sentenced to 6 months in federal prison and to pay a fine of $100,000 and forfeit $500,000. The CEO pleaded guilty to a misdemeanor for his role in causing the introduction of misbranded Suboxone Film into interstate commerce. Thaxter had been the CEO of Indivior for 12 years.
Suboxone Maker Sentenced to Pay $289 Million in Criminal Penalties for Unlawful Marketing of Suboxone
In November of 2020, Indivior Solutions, the maker of Suboxone, was sentenced to pay $289 million in criminal penalties related to previously pleading guilty to improperly marketing Suboxone. The sentence pursuant to a plea agreement was entered by U.S. District Judge James P. Jones of the Western District of Virginia.
$300 Million Suboxone Settlement For Improper Marketing of Suboxone
In April of 2021, Indivior – the maker of Suboxone, agreed to settle claims with all 50 states, the District of Columbia, and Puerto Rico, regarding improper marketing of Suboxone, resulting in improper use of state Medicaid funds for $300 million. Approximately $204 million of the Suboxone settlement will go to Medicaid, with the remainder of the money going to the 50 states, DC, and Puerto Rico. The Suboxone settlement was announced by the California Attorney General, and it was noted that the state of California would get approximately $1.6 million of the Suboxone settlement. It was noted that this Suboxone settlement specifically resolved allegations from 2010 through 2015 that the makers of Suboxone:
- Promoted the sale and use of Suboxone to doctors who were writing Suboxone prescriptions that lacked a legitimate medical purpose, were unsafe, ineffective, and medically unnecessary;
- Knowingly promoted the sale or use of Suboxone Sublingual Film based on false and misleading claims that Suboxone Sublingual Film was less likely to be diverted to and abused than other products, and that children were less likely to be exposed to Suboxone Sublingual Film;
- Fraudulently submitting a petition to the FDA on September 25, 2012, claiming that the Suboxone Tablet had to be discontinued “due to safety concerns” with the Suboxone tablet formulation and took additional steps to fraudulently delay the entry of generic Suboxone in order to improperly control the price of Suboxone.
This $300 million Suboxone settlement also resolves six whistleblower lawsuits against the makers of Suboxone in Virginia and New Jersey federal courts.
$385 Million Suboxone Settlement to Resolve Suboxone Antitrust Multi-District Litigation
In October of 2023, the makers of Suboxone announced they had reached a $385 million Suboxone settlement to resolve the claims brought by the direct purchasers in the In re Suboxone Antitrust Litigation multi-district litigation. The Suboxone trial had been scheduled to begin on October 30, 2023. The Suboxone settlement concluded the Suboxone Antitrust MDL in the United States District Court for the Eastern District of Pennsylvania. It was noted that the $385 million Suboxone settlement would be paid in November 2023 and funded from the company’s existing cash.
Formation of In Re: Suboxone Film Products Liability Litigation (MDL 3092)
On February 2, 2024, the United States Judicial Panel on Multidistrict Litigation (JPML) created In Re: Suboxone (Buprenorphine/Naloxone) Film Products Liability Litigation, known as MDL 3092. The creation of the Suboxone MDL consolidated all personal injury Suboxone lawsuits alleging dental injuries that had been filed in federal courts around the United States into the Northern District of Ohio. At the time Suboxone attorneys moved for a Suboxone MDL, only 15 Suboxone personal injury lawsuits were on file in 5 district courts. By the time the JPML decided to create the Suboxone MDL, the JPML had been notified of an additional 11 Suboxone dental lawsuits in nine districts.
The Suboxone plaintiff lawyers requested centralization of the Suboxone MDL in the Northern District of Ohio and the Suboxone defendants agreed that centralization in the Northern District of Ohio was appropriate. The JPML found the following in deciding to form the Suboxone MDL:
- “these actions involve common questions of fact, and that centralization in the Northern District of Ohio will serve the convenience of the parties and witnesses and promote the just and efficient conduct of this litigation. These actions share complex factual questions arising from the alleged propensity of Suboxone film, which is used for the treatment of opiate addiction, to cause dental erosion and decay. Plaintiffs in all actions allege that defendants designed Suboxone film to be acidic, which they claim leads to dental erosion and decay when the film is dissolved in the mouth (Suboxone previously was available only as an ingestible tablet). Plaintiffs allege that defendants knew, but failed to warn, that Suboxone film causes damage to teeth. The same factual questions regarding general causation, including the mechanism of the alleged injury, are present in all cases. Similarly common are questions surrounding the adequacy of the testing defendants conducted regarding Suboxone film and the sufficiency of warnings regarding dental problems.
In deciding to have the Suboxone MDL in the Northern District of Ohio, the JPML found significant that 13 Suboxone lawsuits were currently pending in the Northern District of Ohio. The Suboxone MDL was assigned to the Honorable J. Philip Calabrese. The JPML noted that centralization of the Suboxone MDL “before the Honorable J. Philip Calabrese allows us to assign this litigation to a jurist who has not yet had the opportunity to preside over an MDL.”
Contact TheLawFirm.com for a free Suboxone lawsuit consultation. Our experienced team is prepared to guide you through your legal options. Call us at 1-888-612-8313 or fill out our contact form. Remember, we stand with you in this fight for justice.
Additional Resources
For more insights:
Call Toll Free To Speak With A Suboxone Sublingual Film Lawyer
1-888-612-8313
Legal Disclaimer: The information in this article is not intended to be used as medical information or diagnosis. The sources of the information presented in the article have been researched and are linked within the article. Please seek out medical advice from a licensed medical professional if you are experiencing a problem with any of the drugs or devices mentioned in this article.
Related Articles
About TLF

TheLawFirm.com is a group of award winning attorneys, paralegals and associates from the legal profession who’s main goal is to educate and represent their clients with the utmost expertise, respect and trust.
We also work closely with a large group of experts from the medical profession so we can draw upon their expertise, in order to present as much accurate information relating to various mass tort and personal injury lawsuits as we can.



